Introduction Many clinical trials these days are global in nature. Patients recruited into the trials, as well as the researchers conducting the trial in their country of origin, usually do not rely on English as their primary language. The importance of accurately translating clinical trials so the data can be gathered, researched, and made available … Learn More
Tag: clinical translation

The impact of language errors in clinical trials can be significant, leading to confusion for the participants and researchers. These frustrating events can lead to dropouts, delays in execution timelines, and even affect the validity of the ultimate trial results. Because of this, regulatory agencies now require that global clinical trials are accurate, consistent, and culturally appropriate in each of the local countries they are administered.
Investing in high-quality transcreation services can be a major differentiator for a successful clinical trial, by ensuring regulatory compliance and improved patient outcomes.
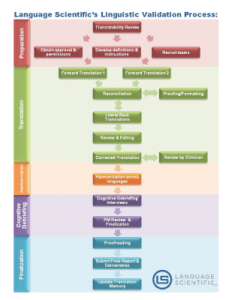
In the realm of clinical research, the accuracy of patient feedback is paramount. This is where Cognitive Debriefing comes into play, particularly in the translation of patient questionnaires. These specialized interviews are a cornerstone of linguistic validation, ensuring that Patient-Reported Outcomes (PROs) are not just linguistically accurate but also culturally relevant and understandable across diverse populations. Cognitive Debriefing is a process embedded within the translation of clinical instruments. When patients from different linguistic backgrounds are required to provide information about their health and treatment experiences, the clarity of each translated questionnaire item is crucial. Cognitive Debriefing allows researchers to evaluate not just the translation’s validity, but also its relevance and accessibility to the target demographic.