If you do not know what it means, linguistic validation and cognitive debriefing may sound complicated and intimidating. Those in clinical research know that linguistic validation and cognitive debriefing are necessary for qualifying an instrument’s validity for use in multinational trials and protecting research data pools. Without linguistic validation and cognitive debriefing, clinical research trials … Learn More
Tag: clinical research
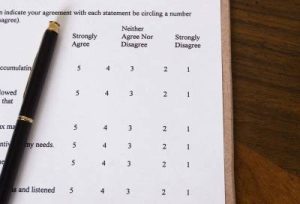
In an earlier blog (What is Linguistic Validation?) we discussed what is involved with linguistic validation; we now turn our attention to explaining cognitive debriefing, sometimes referred to as pilot testing. As stated before, linguistic validation and cognitive debriefing are needed to qualify an instrument’s validity and to protect clinical research data pools. The US Food and … Learn More

There is a debate about bringing the language of Informed Consent Forms (ICFs) down to a level that is more universally understandable. If English speakers struggle to understand the language of Informed Consent Forms, imagine the difficulty that exists for people with limited English proficiency (LEP). The US Food and Drug Administration (FDA) (21 CFR … Learn More

Have you ever been asked to have a back translation and reconciliation done for your forward translation and you weren’t sure what that means or why it was necessary? Back translation and reconciliation services give you additional quality and accuracy assurance for your most sensitive translation and localization projects. Both back translation and reconciliation become important … Learn More