Introduction In today’s rapidly evolving world of medical clinical research, the requirement for precise and reliable translation services is greater than ever. Here’s a link to a Blog titled “Making Medical Translation A Differentiating Factor For Successful Clinical Trials” that delves into this issue in full detail. With the recent advances in Artificial Intelligence (AI), … Learn More
Tag: medical translation

Introduction The European Union Medical Device Regulation (MDR) adopted in April 2017, changed the European legal framework for medical devices and introduced new principal and supportive responsibilities for EMA and for national authorities in the assessment of certain categories of medical products. The aim of the MDR is to ensure patient safety and provide greater transparency and traceability … Learn More

Introduction In a recent blog post titled Complying with European In-Vitro Diagnostic Regulation (IVDR) Language Translation Requirements, we described what this is regulation is and its intent. In summarizing, IVDR mandates that any information accompanying medical devices must be provided in the EU languages accepted in the markets where the device is sold. When enacted … Learn More

The impact of language errors in clinical trials can be significant, leading to confusion for the participants and researchers. These frustrating events can lead to dropouts, delays in execution timelines, and even affect the validity of the ultimate trial results. Because of this, regulatory agencies now require that global clinical trials are accurate, consistent, and culturally appropriate in each of the local countries they are administered.
Investing in high-quality transcreation services can be a major differentiator for a successful clinical trial, by ensuring regulatory compliance and improved patient outcomes.
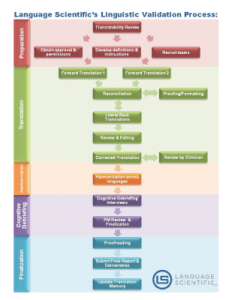
In the realm of clinical research, the accuracy of patient feedback is paramount. This is where Cognitive Debriefing comes into play, particularly in the translation of patient questionnaires. These specialized interviews are a cornerstone of linguistic validation, ensuring that Patient-Reported Outcomes (PROs) are not just linguistically accurate but also culturally relevant and understandable across diverse populations. Cognitive Debriefing is a process embedded within the translation of clinical instruments. When patients from different linguistic backgrounds are required to provide information about their health and treatment experiences, the clarity of each translated questionnaire item is crucial. Cognitive Debriefing allows researchers to evaluate not just the translation’s validity, but also its relevance and accessibility to the target demographic.